
Dedicated To Treating
Life-Threatening
Inflammatory Diseases
Affecting the Pancreas,
Kidney and Lung.
The goal of CalciMedica’s development programs is to explore the therapeutic benefits of CRAC (calcium release-activated calcium) channel inhibitors in inflammatory diseases of the pancreas, kidney, lung and other organs.
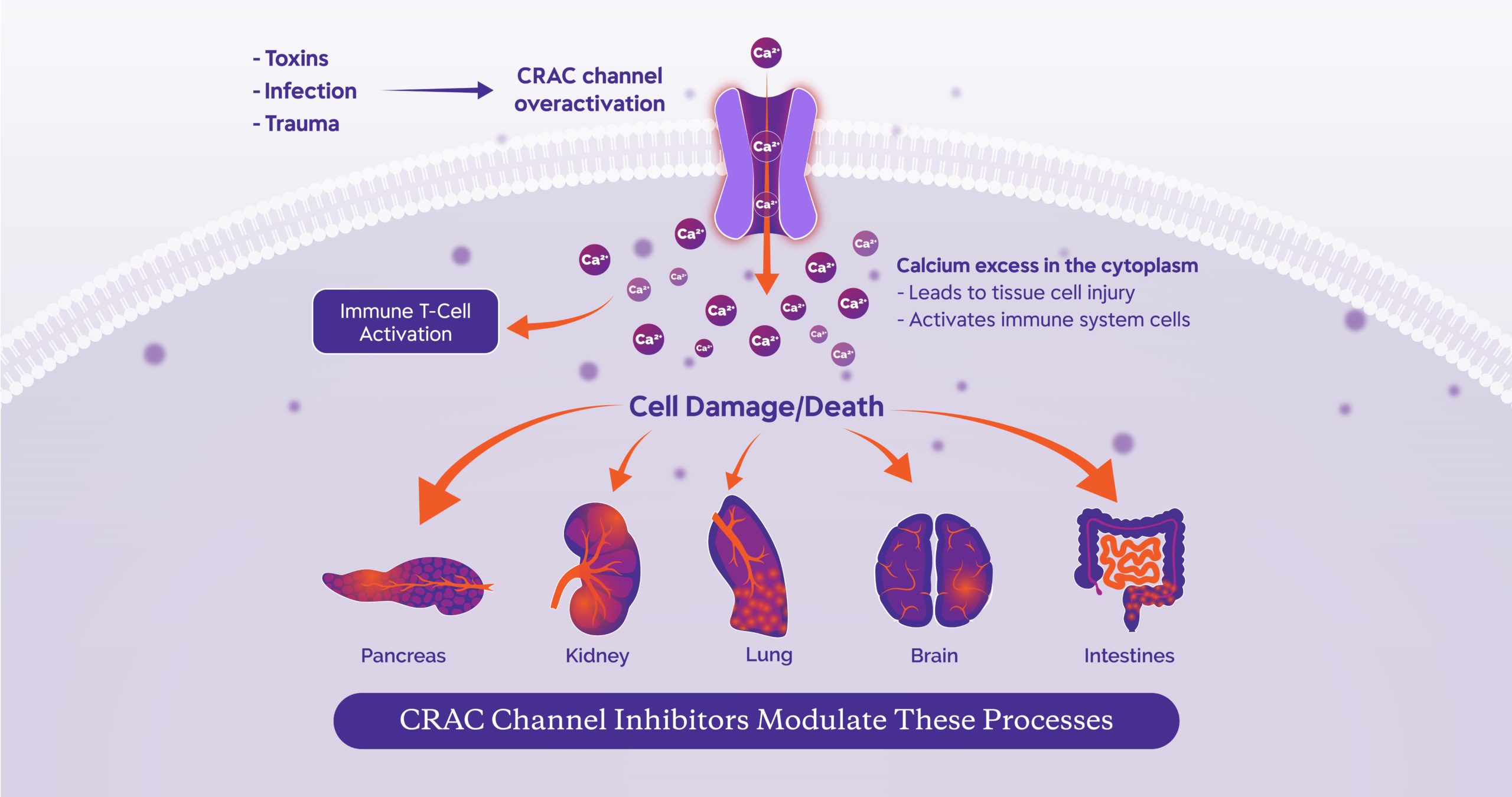
Leading the Way in CRAC Channel Inhibition: We are developing a new class of therapies that target the inhibition of CRAC channels and are designed to modulate the immune response and protect against tissue cell injury.
We Develop Therapies
for Life-threatening
Inflammatory Illnesses.
CalciMedica is developing therapeutics that treat serious
illnesses driven by inflammatory processes and direct
cellular damage. Our CRAC channel inhibitors have the
potential to provide therapeutic benefits in life-threatening
inflammatory diseases for which there are currently no
approved disease-modifying treatments.
Hospitalizations per year in the US, with one third having or developing SIRS, indicative of predicted or severe disease
- Standard of care: Supportive care – IV fluids, pain medication, and nutrition
- Severe complications include pancreatic necrosis, life-threatening distal organ failure, and acute respiratory distress syndrome (ARDS)
- 20-30% mortality rate in patients with severe disease
Children with acute lymphoblastic leukemia (ALL) treated with chemotherapeutic asparaginase in US annually, 7-10% of whom develop AIPT
- Standard of care: Supportive care – IV fluids, pain medication, and nutrition
- >50% of pediatric AIPT patients develop pancreatic necrosis and/or pancreatic pseudocysts
- AIPT requires that asparaginase treatment be halted beyond this there can be other short- or long-term consequences
30% of 3.7MM patients hospitalized annually in the US with AKI are Stage 2 and Stage 3
- Standard of Care: Supportive care Treatment of complications; dialysis
- Disease can progress to:
- Chronic kidney disease
- End stage renal disease
- Eventual Death
Patients per year in the U.S.
3M Patients per year globally
- Standard of care: Oxygen and fluid management; invasive mechanical ventilation
- 10% of intensive care unit admissions worldwide each year. 75-80% moderate or severe. Life-threatening complications including organ damage or organ failure
- 30-40% mortality rate. Leads to 75,000 deaths annually in the U.S

Auxora™
Auxora™, a proprietary, intravenous formulation of a small
molecule calcium release-activated calcium (CRAC) channel
inhibitor, is in development for the treatment of acute
pancreatitis (AP) with SIRS and asparaginase-induced pancreatic
toxicity (AIPT), for which there are no currently approved
therapies.






A Leadership Team
Dedicated to Science and
Innovation.
The CalciMedica team is comprised of scientists, physicians,
drug development experts and entrepreneurs dedicated to
the development of CRAC channel inhibitors for acute and
chronic inflammatory conditions.










“Our focus at CalciMedica is on developing and bringing to market an entirely new class of therapies – CRAC channel inhibitors – to fight life-threatening inflammatory diseases of the pancreas, kidney, and lung. We are dedicated to addressing the high unmet need for safe and effective therapies to benefit patients with acute illnesses for which there are currently no approved treatments.”


